Looking at the sides of molecules
Shinjae Nam and Alfred Weymouth discuss results in front of the low-temperature microscope where data were collected. © A. J. Weymouth
Lateral force microscopy reveals previously unseen hydrogen atoms.
Study published in the journal “Proceedings of the National Academy of Sciences”.
Researchers at the University of Regensburg and the Graz University of Technology have shown that hydrogen atoms at the sides of molecules lying on a surface can directly be seen. The study, published in the journal “Proceedings of the National Academy of Sciences”, describes that by looking beside the molecules, the position and presence of the previously-hidden hydrogen atoms could be revealed.
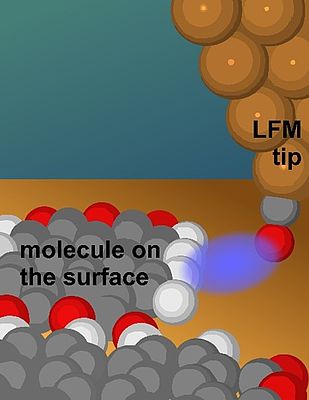
Artists rendition of a LFM tip coming close to the side of a molecule, where it is sensitive to the terminal H-atoms. © A. J. Weymouth
Hydrogen atoms situated at the edges of molecules affect many properties of those molecules, including how they interact with other molecules. Hydrogen bonds are one of the most common forms of molecular interactions, in which a positively-charged hydrogen atom at the side of a molecule is attracted to a negative atom in a neighbouring molecule. Hydrogen bonds are of great importance in the field of on-surface synthesis, in which molecules are first absorbed onto a surface and then react with each other. But despite their significance, direct observations of these small yet important atoms have been elusive.
To visualize the sides of molecules, researchers employed a specialized technique derived from Atomic Force Microscopy (AFM). In AFM, a sharp tip is brought close to a surface, and the forces on the tip are recorded as it moves over the surface. Previous AFM experiments focussed on the vertical component of force and had not revealed the hydrogen atoms at the sides of molecules.
To overcome this limitation, researchers employed Lateral Force Microscopy (LFM), which measures the horizontal forces exerted on the AFM tip. PD Dr Alfred J. Weymouth from the working group of Prof. Dr Franz J. Gießibl, holder of the Chair of Quantum Nanoscience at the UR, is a leading expert in the field of LFM. He highlighted its unique capabilities, stating, “despite the fact that it is not widely used, LFM offers several advantages over conventional AFM, including exceptional distance sensitivity, enabling the extraction of physical parameters from a single image, and the ability to quantify frictional forces by sliding a single atom across chemical bonds.”
By measuring the lateral force exerted on the AFM tip at the edges of the molecules, Dr Weymouth and coworkers were able to directly visualize the hydrogen atoms. The raw data from the experiments could be compared directly to theoretical calculations, providing a deeper understanding of the atomic interactions at play. While atom-atom interactions are often modeled using simplified distance-dependent functions, comparing these models to the experimental data revealed the limitations of these approximations, highlighting the importance of incorporating additional factors into these theoretical frameworks. This insight is valuable for both AFM and LFM investigations, as it allows researchers to refine their understanding of basic atomic interactions.
The ability to directly observe hydrogen atoms marks a significant breakthrough for researchers, providing a powerful tool to elucidate the intricate mechanisms and intermediate steps of on-surface chemical reactions. This advancement holds immense potential for accelerating progress in various fields, including surface catalysis and molecular interactions within the human body. The development of this novel technique represents a significant step forward in our understanding of the microscopic world, opening up new avenues for research and innovation. By directly visualizing the behavior of hydrogen atoms, researchers can gain deeper insights into the fundamental processes that govern the interactions of molecules, paving the way for transformative advancements in various fields.
About the University of Regensburg and the Graz University of Technology
The University of Regensburg’s Department of Physics is one of the leading research institutions in Germany with a strong focus on condensed matter physics. Professor Gießibl runs the Quantum Nanoscience group, where the experimental work was performed. Professor Hofmann runs the Simulation-Driven Material Discovery Group at the Graz University of Technology, where the theoretical calculations were performed.
Original publication
Nam, Shinjae; Riegel, Elisabeth; Hörmann, Lukas; Hofmann, Oliver T.; Gretz, Oliver; Weymouth, Alfred J.; Giessibl, Franz J.: „Exploring in-plane interactions beside an adsorbed molecule with lateral force microscopy” (2024), Proceedings of the National Academy of Sciences, doi:10.1073/pnas.2311059120;
https://www.pnas.org/doi/abs/10.1073/pnas.2311059120
Media Contact
All latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…