Identifying the ’signatures’ of protons in water
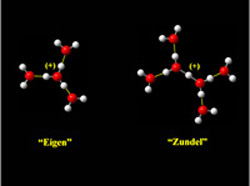
Eigen and Zundel models for proton shared by water molecules
Free protons from acids associate with 1, 2 or 3 molecules of water and the structures can be identified by unique infrared laser spectrum signatures, according to a report in Science by Yale professor of chemistry Mark A. Johnson and his collaborators at Yale, the University of Pittsburgh and the University of Georgia.
Acids yielding free protons are common in biological and chemical systems and the measurement of pH to determine acidity of an aqueous solution is a simple, standard procedure. However, it has not been as easy to determine where the liberated protons are located and how they interact with water molecules.
The scientists tackled these questions using infra-red laser light, at much lower energies than were previously accessible, to monitor how the vibration profile changes when a proton is associated with two to eleven water molecules.
The researchers first established a spectral signature for the symmetrically hydrated Eigen cation, which has a minimum energy (H3O)+ ion core and three associated “dangling” water molecules. As they successively added or subtracted water molecules and compared the spectral signatures, they mimicked water fluctuations.
“Surprisingly large spectral shifts are driven by small changes in the hydration environment,” said Johnson. “Although previous work anticipated a change from Zundel to Eigen structures as you progress from 8 to 9 water molecules, the change in the low energy bands here is dramatic. The profile for the 9-membered cluster is much like bulk water, but then the 10-membered cluster is again simpler.”
The study shows that the proton associated with the Eigen cation undergoes vibrations highest in energy because it supports the greatest distribution of charge, that is, over three H atoms. As different numbers of water molecules surround the H3O+ core, the excess charge can become more localized onto two or even one of the H atoms, causing substantial, size-dependent shifts in the spectral signature of the excess proton. This extreme response to breaking symmetry is consistent with Zundel’s model of the excess proton being a highly polarizable species.
“The basic point is that the proton is a moving target, rapidly switching its character from one species to the next according to how many water molecules it is associated with,” said Johnson. “Now that the spectral signatures of various local environments in water are known, the big question left is how this all comes together as we continue to grow crystals toward bulk water (ice).”
Media Contact
More Information:
http://www.yale.eduAll latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

How marine worms regenerate lost body parts
The return of cells to a stem cell-like state as the key to regeneration. Many living organisms are able to regenerate damaged or lost tissue, but why some are particularly…

Nano-scale molecular detective
New on-chip device uses exotic light rays in 2D material to detect molecules. Researchers have developed a highly sensitive detector for identifying molecules via their infrared vibrational “fingerprint”. Published in Nature…

Novel CAR T-cell therapy
… demonstrates efficacy and safety in preclinical models of HER2-positive solid tumors. The p95HER2 protein is found expressed in one third of HER2+ tumors, which represent 4% of all tumors….



