Unravelling how ions move in phosphate glass
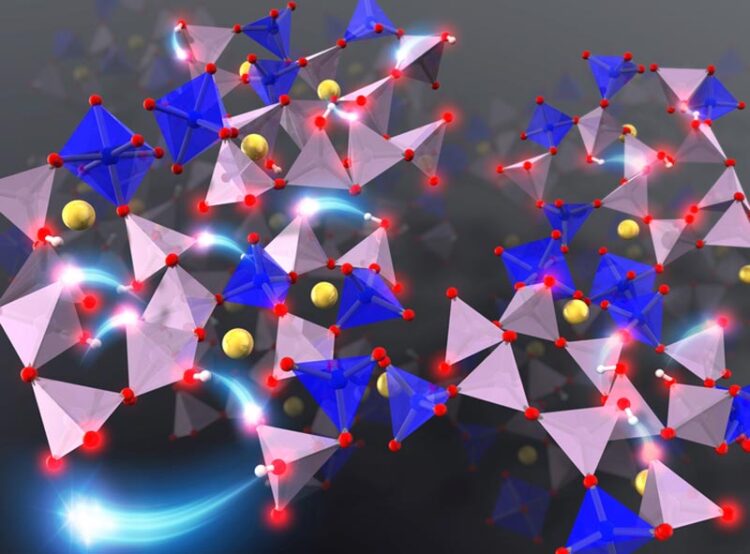
Investigating the microscopic diffusion mechanism of protons and sodium ions in phosphate glasses via first-principles molecular dynamics simulation indicates the key role of the morphology of the phosphate network structure on the diffusion of ions.
Image Credit: Tomoyuki Tamura from Nagoya Institute of Technology
Through the looking glass…
In a new study based on the theoretical computation of atomic structures, researchers determine the mechanisms of ion diffusion in phosphate glass.
Phosphate glass is a versatile compound that has generated interest for its use in fuel cells and as biomaterials for supplying therapeutic ions. P2O5–the compound that forms the structural network of phosphate glass, is made up of phosphorus, an element that can adopt many different bonding configurations in combination with oxygen.
The physicochemical properties crucial for the real-life applicability of phosphate glass—for instance, the hydration reaction dictating how quickly a phosphate glass-based biomaterial will dissolve inside the body—depends on the diffusion of ions into the glass. Thus, to improve the physicochemical properties of phosphate glasses, it is important to understand the relationship between the structure and ion diffusion. However, studying such interactions at the atomic level is extremely difficult, prompting scientists to search for a suitable approach to illuminate the details of the ion diffusion process.
Recently, a team of researchers from Nagoya Institute of Technology, led by Dr. Tomoyuki Tamura, has theoretically deciphered the ion diffusion mechanism involved in the hydration reaction process of phosphate glasses. Their study has been published in the Physical Chemistry Chemical Physics journal.
In fully connected P2O5-based phosphate glass, three of the oxygen atoms in each phosphate unit are bonded to neighboring phosphorous atoms. To study the dynamics of ions in the phosphate glass during the hydration process, the researchers used a model made of phosphates with QP2 and QP3 morphologies, that contain two and three bridging oxygens per PO4 tetrahedron, respectively, along with six coordinated silicon structures.
The researchers implemented a theoretical computational approach known as “first-principles molecular dynamic (MD) simulation” to investigate the diffusion of proton and sodium ions into the glass. Explaining the rationale for their unconventional approach, Dr. Tamura says, “First-principles MD simulation enabled us to assume the initial stage of water infiltrating and diffusing into silicophosphate glass and elucidate the diffusion of protons and inorganic ions for the first time.”
Based on their observation, the researchers proposed a mechanism where the protons “hop” and are adsorbed onto the non-bridging oxygen or “dangling” oxygen atom of nearby phosphates through hydrogen bonds. However, in the phosphate glass model they used, the QP2 phosphate units contributed more strongly to the diffusion of protons than the QP3 phosphate units. Thus, they found that the morphology of the phosphate network structure, or the “skeleton” of the glass, greatly affects the diffusion of ions. They also noticed that when a sodium ion was present in the vicinity, the adsorption of a proton onto a QP2 phosphate unit weakened the electrostatic interaction between sodium and oxygen ions, inducing the chain diffusion of sodium ions.
The demand for new biomaterials for effective prevention and treatment is on the rise, and phosphate glasses are well-poised to fulfill this growing need. A large proportion of the population, comprising both elderly and younger people, suffers from diseases related to bone and muscle weaknesses. As Dr. Tamura surmises, “Water-soluble silicophosphate glass is a promising candidate for supplying drugs or inorganic ions that promote tissue regeneration, and our study takes the research in glass technology one step nearer towards realizing the goal.”
Thus, the researchers’ novel insights are bound to have profound real-life impact and lead to breakthroughs in research on fuel cells and bioresorbable materials!
About Nagoya Institute of Technology, Japan
Nagoya Institute of Technology (NITech) is a respected engineering institute located in Nagoya, Japan. Established in 1949, the university aims to create a better society by providing global education and conducting cutting-edge research in various fields of science and technology. To this end, NITech provides a nurturing environment for students, teachers, and academicians to help them convert scientific skills into practical applications. Having recently established new departments and the “Creative Engineering Program,” a 6-year integrated undergraduate and graduate course, NITech strives to continually grow as a university. With a mission to “conduct education and research with pride and sincerity, in order to contribute to society,” NITech actively undertakes a wide range of research from basic to applied science.
Website: https://www.nitech.ac.jp/eng/index.html
About Dr. Tomoyuki Tamura from Nagoya Institute of Technology, Japan
Dr. Tomoyuki Tamura is an Associate Professor at Nagoya Institute of Technology, Japan. Dr. Tamura is an active member of the research group of Department of Physical Science and Engineering. He earned his doctoral degree from The University of Tokyo Graduate School, Division of Engineering in 2004 and then continued his career as a researcher. His areas of interest include machine-learning based first-principles simulations, machine-learning based modeling, computational design of ion-conductive materials, and grain-boundary properties. As an expert in his field, Dr. Tamura has authored more than 50 scientific publications in reputed research journals.
About Dr. Toshihiro Kasuga from Nagoya Institute of Technology, Japan
Dr. Toshihiro Kasuga is a Professor at the Department of Life Science and Applied Chemistry, Nagoya Institute of Technology, Japan. He received his Dr. Eng. from Osaka University in 1993. Dr. Kasuga’s areas of expertise include biomaterial science and engineering, inorganic amorphous materials, and phosphate-based functional materials. He has authored several textbooks and over 300 papers on these topics, which have been published in journals of international repute.
Journal: Physical Chemistry Chemical Physics
DOI: 10.1039/d1cp01646f
Method of Research: Experimental study
Subject of Research: Not applicable
Article Title: Diffusion of protons and sodium ions in silicophosphate glasses: insight based on first-principles molecular dynamic simulations
Article Publication Date: 22-Jun-2021
COI Statement: The authors have declared no competing interest.
Media Contact
Azusa Yabugami
yabugami.azusa@nitech.ac.jp
Office: 81-527-355-091
All latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

Largest magnetic anisotropy of a molecule measured at BESSY II
At the Berlin synchrotron radiation source BESSY II, the largest magnetic anisotropy of a single molecule ever measured experimentally has been determined. The larger this anisotropy is, the better a…

Breaking boundaries: Researchers isolate quantum coherence in classical light systems
LSU quantum researchers uncover hidden quantum behaviors within classical light, which could make quantum technologies robust. Understanding the boundary between classical and quantum physics has long been a central question…

MRI-first strategy for prostate cancer detection proves to be safe
Active monitoring is a sufficiently safe option when prostate MRI findings are negative. There are several strategies for the early detection of prostate cancer. The first step is often a…



