Newly developed compound may enable sustainable, cost-effective, large-scale energy storage
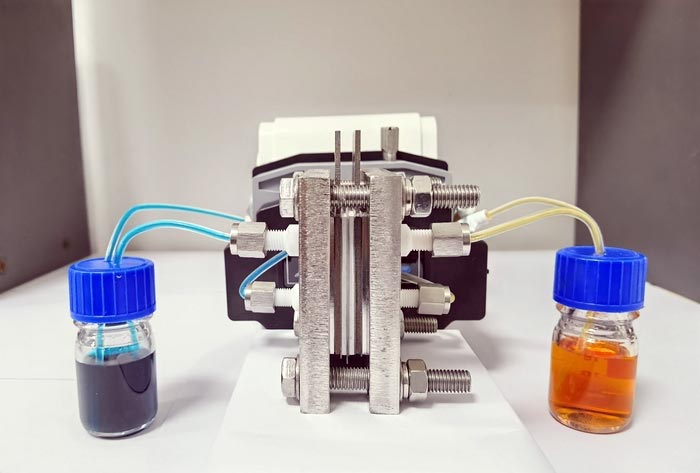
Photo of aqueous redox flow battery
Credit: Liwen Wang, South China University of Technology
To produce a cost-effective redox flow battery, researchers based at the South China University of Technology have synthesized a molecular compound that serves as a low-cost electrolyte, enabling a stable flow battery that retains 99.98% capacity per cycle. They published their approach on August 14 in the Energy Material Advances.
Comprising two tanks of opposing liquid electrolytes, the battery pumps the positive and negative liquids along a membrane separator sandwiched between electrodes, facilitating ion exchanges to produce energy. Significant work has been dedicated to developing the negative electrolyte liquid, while the positive electrolyte liquid has received less attention, according to corresponding author Zhenxing Liang, professor in the Key Laboratory of Fuel Cell Technology of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology.
“Aqueous redox flow batteries can realize the stable electrical output for using unsteady solar and wind energy, and they have been recognized as a promising large-scale energy storage technology,” Liang said. “Electroactive organic merit of element abundance, low cost and flexible molecular control over the electrochemical features for both positive and negative electrolytes are regarded as key to developing next-generation redox flow batteries.”
Liang and his team focused on TEMPO, a chemical compound with easily reversed oxidation states and high potential for energy, a desired quality in positive electrolytes.
“However, TEMPO cannot be directly applied to aqueous redox flow batteries due to the high hydrophobicity of the molecular skeleton,” Liang said, explaining that TEMPO, left unmodified, will not dissolve in the liquid needed to facilitate the energy exchange in the flow batteries. “We developed a strategy to functionalize TEMPO with viologen, an organic compound that has highly reversible redox reactions, to improve TEMPO’s hydrophilicity.”
According to Liang, viologen is highly soluble in water, which increases TEMPO’s ability to dissolve in water. Viologen also chemically withdraws electrons from atomic partners, which elevates its potential to change its oxidative state. Viologen is also a salt, which endows TEMPO with what Liang calls “a decent conductivity” in an aqueous solution.
When the synthesized viologen-modified TEMPO was tested in a flow battery, the researchers found that the battery retained capacity of 99.98% per cycle, meaning the battery could hold nearly all its stored energy when not in active use.
“This work overcomes the disadvantages of TEMPO by viologen-functionalization and realizes its application in aqueous redox flow battery,” Liang said. “The molecular design concept provides a strategy for novel organic electroactive materials and lays a foundation for the application of aqueous organic flow battery.”
Other contributors include Shuzhi Hu, Liwen Wang, Xianzhi Yuan, Zhipeng Xiang, Mingbao Huange, Peng Luo, Yufeng Liu and Zhiyong Fu, all with the Key Laboratory of Fuel Cell Technology of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology. Hu is also affiliated with the School of Materials Science and Engineering, Sun Yat-sen University.
The National Natural Science Foundation of China (21975081 and 21905114) supported this research.
Reference
Authors: Shuzhi Hu,1,2 Liwen Wang,1 Xianzhi Yuan,1 Zhipeng Xiang,1 Mingbao Huang,1 Peng Luo,1 Yufeng Liu,1 Zhiyong Fu,1 and Zhenxing Liang1
Title of original paper: Viologen-Decorated TEMPO for Neutral Aqueous Organic Redox Flow Batteries
Journal: Energy Material Advances
DOI: 10.34133/2021/9795237
Affiliations:
1Key Laboratory of Fuel Cell Technology of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510641, China
2School of Materials Science and Engineering, Sun Yat-sen University, Guangzhou 510275, China
About Dr. Zhengxing Liang
Zhenxing Liang is currently a professor of South China University of Technology.
Research Interests: Electrocatalysis by SECM and other electrochemical methods, Nanostructured carbon materials, Polymer electrolyte membranefuel cells, Li-ion battery & redox flow battery.
Journal: Energy Material Advances
DOI: 10.34133/2021/9795237
Method of Research: Commentary/editorial
Subject of Research: Not applicable
Article Title: Viologen-Decorated TEMPO for Neutral Aqueous Organic Redox Flow Batteries
Article Publication Date: 14-Aug-2021
COI Statement: The authors declare no conflict of interest.
Media Contact
Ning Xu
Beijing Institute of Technology Press Co., Ltd
xuning1907@foxmail.com
Original Source
https://spj.sciencemag.org/journals/energymatadv/2021/9795237/
All latest news from the category: Power and Electrical Engineering
This topic covers issues related to energy generation, conversion, transportation and consumption and how the industry is addressing the challenge of energy efficiency in general.
innovations-report provides in-depth and informative reports and articles on subjects ranging from wind energy, fuel cell technology, solar energy, geothermal energy, petroleum, gas, nuclear engineering, alternative energy and energy efficiency to fusion, hydrogen and superconductor technologies.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



