Research into the chemistry of environmentally friendly power generation
Researchers of the Faculty of Science at the ELTE Eötvös Loránd University investigate the properties of methane, the most important component of natural gas. Accurate knowledge of the ignition of methaneair mixtures will help to increase the efficiency and reduce the environmental impact of heating and power generation, and could also lead to further developments in industrial safety, chemical and energy engineering.
Photo: ELTE Eötvös Loránd University
Accurate knowledge of the ignition of methane-air mixtures will help to increase the efficiency and reduce the environmental impact of heating and power generation.
Researchers of the Faculty of Science at the ELTE Eötvös Loránd University investigate the properties of methane, the most important component of natural gas. Accurate knowledge of the ignition of methane-air mixtures will help to increase the efficiency and reduce the environmental impact of heating and power generation, and could also lead to further developments in industrial safety, chemical and energy engineering.
In Hungary, the majority of households use natural gas for heating:
it is one of the most environmentally friendly ways of keeping our homes warm.
However, natural gas also plays an important role in electricity generation. Solar and wind power generations have high fluctuations, and an effective way to compensate for this is to use gas turbines and gas engines based on natural gas combustion, which can quickly make up for the missing electricity.
A significant proportion of natural gas is methane to varying degrees depending on the area; in Hungary natural gas contains nearly ninety-seven per cent methane. In order utilize this colourless, odourless, flammable substance more efficiently in environmentally friendly power generation, it is important to study the explosion of methane-air mixtures, the spread of flame in gas engines, and the formation of harmful pollutants during the combustion of natural gas.
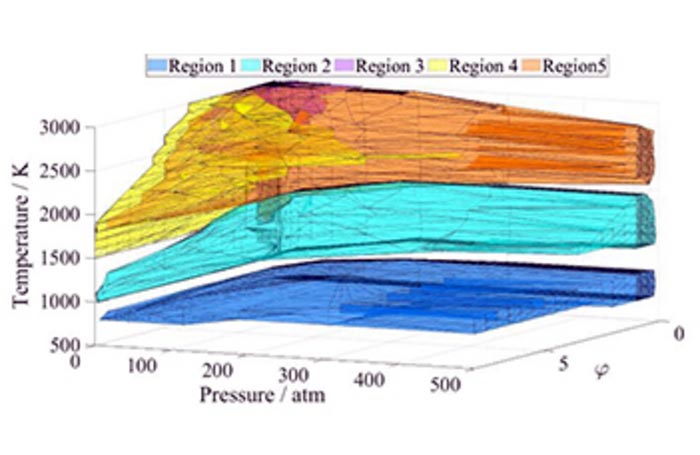
The Chemical Kinetics Laboratory of the Institute of Chemistry and the Department of Applied Analysis and Computational Mathematics of the Institute of Mathematics at ELTE – Éva Valkó, Máté Papp, Peng Zhang and Tamás Turányi – investigated the ignition of methane-air mixtures based on a detailed reaction kinetics model. The sensitivity vector of the ignition time was calculated by varying the initial temperature (T), pressure (p) and methane-to-air equivalence ratio (ϕ) over a wide range. A cluster analysis of the resulting more than 14 thousand sensitivity vectors showed that five domains can be identified in the (T, p, ϕ) space, and that in each domain a different set of chemical reactions leads to methane ignition. The results were published in the Proceedings of the Combustion Institute.
“Operation of gas engines include compressing a mixture of natural gas and air rapidly to reach a given temperature and pressure. The time to explosion for this high-pressure, hot gas mixture is critical.
If a reliable computer model based on a detailed reaction mechanism is available, it can be used to determine whether or not an explosion will occur,”
says Prof. Tamás Turányi, the head of the research.
For years, Tamás Turányi’s research group has also been investigating the so-called e-fuels, which can overcome the above mentioned limitation of renewable energy production, namely the large fluctuations in the amount of electricity produced. In e-fuels, the energy content of excess electricity can be stored and then converted back into electricity. One such fuel is ammonia, which has the advantage of being produced using mature technology and is easy to transport and store.
DOI: 10.1016/j.proci.2022.07.186
Article Title: Identification of homogeneous chemical kinetic regimes of methane-air ignition
Media Contact
Sara Bohm
Eötvös Loránd University (ELTE), Faculty of Science
sara.bohm@ttk.elte.hu
Office: 36-205-210-454
All latest news from the category: Power and Electrical Engineering
This topic covers issues related to energy generation, conversion, transportation and consumption and how the industry is addressing the challenge of energy efficiency in general.
innovations-report provides in-depth and informative reports and articles on subjects ranging from wind energy, fuel cell technology, solar energy, geothermal energy, petroleum, gas, nuclear engineering, alternative energy and energy efficiency to fusion, hydrogen and superconductor technologies.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…



