Copper oxide photocathodes: laser experiment reveals location of efficiency loss
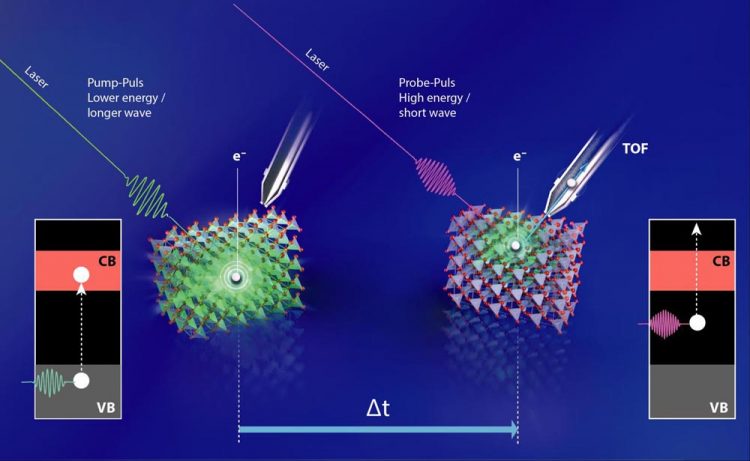
A green laser pulse initially excites the electrons in the Cu2O; just fractions of a second later, a second laser pulse (UV light) probes the energy of the excited electron. Credit: M. Kuensting/HZB
Copper oxide has a band gap of 2 electron volts, which matches up very well with the energy spectrum of sunlight. Perfect copper oxide crystals should theoretically be able to provide a voltage close to 1,5 volts when illuminated with light.
The material would thus be perfect as the top-most absorber in a photoelectrochemical tandem cell for water splitting. A solar-to-hydrogen energy conversion efficiency of up to 18 per cent should be achievable.
However, the actual values for the photovoltage lie considerably below that value, insufficient to make copper oxide an efficient photocathode in a tandem cell for water splitting. Up to now, loss processes near the surface or at boundary layers have been mainly held responsible for this.
A team at the HZB Institute for Solar Fuels has now taken a closer look at these processes. The group received high-quality Cu2O single crystals from colleagues at the renowned California Institute of Technology (Caltech), then vapour-deposited an extremely thin, transparent layer of platinum on them.
This platinum layer acts as a catalyst and increases the efficiency of water splitting. They examined these samples in the femtosecond laser laboratory (1 fs = 10-15 s) at the HZB to learn what processes lead to the loss of charge carriers and in particular whether these losses occur in the interior of the single crystals or at the interface with the platinum.
A green laser pulse initially excited the electrons in the Cu2O; just fractions of a second later, a second laser pulse (UV light) measured the energy of the excited electron. The team was then able to identify the main mechanism of photovoltage losses through this time-resolved two-photon photon emission spectroscopy (tr-2PPE).
“We observed that the excited electrons were very quickly bound in defect states that exist in large numbers in the band gap itself”, reports first author Mario Borgwardt, who is now continuing his work as a Humboldt fellow at Lawrence Berkeley National Laboratory in the USA.
The coordinator of the study, Dennis Friedrich, explains: “This happens on a time scale of less than one picosecond (1 ps = 10-12 s), i.e. extremely fast, especially compared to the time interval charge carriers need to diffuse from the interior of the crystalline material to the surface.”
“We have very powerful experimental methods at the femtosecond laser laboratory of the HZB for analysing energy and dynamics of photo-excited electrons in semiconductors. We were able to show for copper oxide that the losses hardly occur at the interfaces with platinum, but instead in the crystal itself”, says Rainer Eichberger, initiator of the study and head of femtosecond spectroscopy lab.
“These new insights are our first contribution to the UniSysCat Excellence Cluster at the Technische Universität Berlin, in which we are a partner”, emphasises Roel van de Krol, who heads the HZB Institute for Solar Fuels. UniSysCat focusses on catalytic processes that take place over very diverse time scales: while charge carriers react extremely quickly to excitations by light (femtoseconds to picoseconds), chemical processes such as (electro)catalysis require many orders of magnitude more time (milliseconds). An efficient photochemical conversion requires that both processes be optimised together. The current results that have now been published in the renowned journal Nature Communications are an important step in this direction.
Media Contact
All latest news from the category: Process Engineering
This special field revolves around processes for modifying material properties (milling, cooling), composition (filtration, distillation) and type (oxidation, hydration).
Valuable information is available on a broad range of technologies including material separation, laser processes, measuring techniques and robot engineering in addition to testing methods and coating and materials analysis processes.
Newest articles

Pinpointing hydrogen isotopes in titanium hydride nanofilms
Although it is the smallest and lightest atom, hydrogen can have a big impact by infiltrating other materials and affecting their properties, such as superconductivity and metal-insulator-transitions. Now, researchers from…

A new way of entangling light and sound
For a wide variety of emerging quantum technologies, such as secure quantum communications and quantum computing, quantum entanglement is a prerequisite. Scientists at the Max-Planck-Institute for the Science of Light…

Telescope for NASA’s Roman Mission complete, delivered to Goddard
NASA’s Nancy Grace Roman Space Telescope is one giant step closer to unlocking the mysteries of the universe. The mission has now received its final major delivery: the Optical Telescope…



