DuPont at Medtec Europe 2011: High performance polymers for use in medical devices
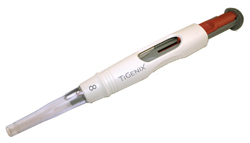
Image: TiGenix <br>The ChondroMimetic™ from TiGenix NV is marketed as a procedure pack with the collagen implant preloaded in an accurate, easy-to-use arthroscopic delivery device. Specialty health care polymers from DuPont facilitate the smooth and accurate delivery of the implant for the user, whilst providing the material regulatory compliance for the manufacturer.<br>
The availability of these specialty health care grades broadens the choice of high performance polymers with healthcare specific manufacturing control and regulatory support. These grades are intended to support healthcare companies which lead medical progress through new and innovative devices.
This is most recently reflected by the adoption of a Special Control (SC) grade of DuPont™ Zytel® nylon for components of an accurate and easy-to-use collagen implant delivery device. The functional performance of the DuPont material and its regulatory compliance are key factors in its specification.
The ChondroMimetic™ from TiGenix NV (Leuven, Belgium) is a CE-marked collagen based implant for the treatment of small osteochondral (cartilage and underlying bone) defects. The implant supports the body's natural repair mechanisms to encourage the simultaneous repair of both articular cartilage and the bone to which it is attached. The product, which was officially launched in Europe during the 9th World congress of International Cartilage Repair Society in October 2010, is marketed as a procedure pack with the collagen implant preloaded in an accurate, easy-to-use arthroscopic delivery device.
The device consists of several polymer components, including a white outer-casing made from ABS and a translucent polycarbonate delivery tube at its tip, which allows visual progress of the implant delivery to be maintained during the surgical procedure. Significantly, the two components made from Zytel® SC – the plunger and compressor fingers – come into direct contact with the implant and are key to holding it in place and inserting it smoothly and accurately into the defect site. The DuPont material was specifically chosen for the stiffness and low-friction behaviour of the plunger as well as providing the precise flexibility of the compressor fingers.
“We specified a Special Control grade of Zytel® nylon based on the advice that the material comes with the required level of manufacturing control, testing and regulatory support for use in our delivery device,” explains Suhaib Hassan, product manager at TiGenix. “For instance, the device is required to meet the requirements of ISO 10993, which includes testing to support the storage of the implant in contact with the plastic parts of the delivery device for its entire shelf life.”
DuPont: the material supplier of choice to the health care segment
DuPont offers one of the broadest portfolios of engineering plastics and thermoplastic elastomers for medical devices, surgical devices and for diagnostic or pharmaceutical manufacturing equipment. The DuPont health care product offering provides food agency compliance (EMSA and FDA), ISO 10993-5 and -11 compliance as well as USP Class VI compliance. Speciality health care products are manufactured following GMP.
The specialty health care grades with regulatory support are available in two versions, each reflecting different levels of manufacturing control and material testing. Customers work with DuPont to identify the version that meets the needs of their specific application. Sixteen of the products are available as “special control” grades that meet very high standards of manufacturing consistency important for a wide range of non-implantable medical products. Twelve of the grades are available in “premium control” versions meeting requirements for even more demanding manufacturing controls, broader regulatory support, more testing, DMF access and the highest level of inspection.
About TiGenix. Based in Leuven, Belgium, TiGenix NV (NYSE Euronext Brussels: TIG) is a public biomedical company that focuses on ‘Regenerating Motion’. The company is exploiting the power of Regenerative Medicine to develop durable treatments, validated through controlled clinical trials, for damaged and diseased skeletal tissues. TiGenix now has two products approved for marketing and sales in Europe: ChondroCelect®, the company’s lead product for cartilage regeneration in the knee, is the first cell-based product that successfully completed the entire development track from research, over clinical development to central European registration as a medicinal product. ChondroCelect consists of characterized cultured chondrocytes derived from the patient’s own cartilage and is used for autologous chondrocyte implantation (ACI), a surgical procedure to treat cartilage defects. ChondroCelect received European Marketing Authorization as the first Advanced Therapy Medicinal Product.
ChondroMimetic™ is an off-the-shelf, collagen based implant for the treatment of small osteochondral (cartilage and underlying bone) defects. ChondroMimetic received CE-mark approval for the treatment of small chondral and subchondral lesions. It will be marketed as a procedure pack with the collagen implant preloaded in an accurate, easy to use delivery device.
The company exploits a proprietary (stem) cell and biomaterials platform, which will continue to generate candidate products that address specific musculoskeletal problems. With a fully operational sales team in place, TiGenix is ready for commercial expansion and reinforcement of its leading position in Europe in the regenerative medicine field.
The DuPont Performance Polymers business manufactures and sells Crastin® PBT and Rynite® PET thermoplastic polyester resins, Delrin® acetal resins, Hytrel® thermoplastic polyester elastomers, DuPont™ ETPV engineering thermoplastic vulcanizates, Minlon® mineral reinforced nylon resins, Neoprene polychloroprene, Tynex® filaments, Vespel® parts and shapes, Sorona® EP, Kalrez® perfluoroelastomer parts, Vamac® ethylene acrylic elastomers (AEM), Viton® fluoroelastomers, Viton® FreeFlow™ processing aids, Zytel® nylon resins, Zytel® PLUS nylon resins and Zytel® HTN high-performance polyamides. These products serve global markets in the aerospace, appliance, automotive, consumer, electrical, electronic, healthcare, industrial, sporting goods and many other diversified industries.
DuPont (www.dupont.com) is a science-based products and services company. Founded in 1802, DuPont puts science to work by creating sustainable solutions essential to a better, safer, healthier life for people everywhere. Operating in more than 90 countries and regions, DuPont offers a wide range of innovative products and services for markets including agriculture and food; building and construction; communications; and transportation.
The DuPont Oval Logo, DuPont™, The miracles of science™ and Zytel® are registered trademarks or trademarks of E.I. du Pont de Nemours and Company or its affiliates.
ChondroCelect® and ChondroMimetic™ are registered trademarks or trademarks of TiGenix NV.
Editorial Contact:
Rémi Daneyrole
Tel.: +41 (0)22 717 54 19
Fax: +41 (0)22 580 22 45
Remi.daneyrole@dupont.com
Media Contact
More Information:
http://www.dupont.comAll latest news from the category: Trade Fair News
Newest articles

Largest magnetic anisotropy of a molecule measured at BESSY II
At the Berlin synchrotron radiation source BESSY II, the largest magnetic anisotropy of a single molecule ever measured experimentally has been determined. The larger this anisotropy is, the better a…

Breaking boundaries: Researchers isolate quantum coherence in classical light systems
LSU quantum researchers uncover hidden quantum behaviors within classical light, which could make quantum technologies robust. Understanding the boundary between classical and quantum physics has long been a central question…

MRI-first strategy for prostate cancer detection proves to be safe
Active monitoring is a sufficiently safe option when prostate MRI findings are negative. There are several strategies for the early detection of prostate cancer. The first step is often a…



